Page 26 - 22-0424
P. 26
The International Journal of the Royal Society of Thailand
Volume XI - 2019
by combining the vaccine with antimalarial prophylactic drugs aiming to increase
the duration of malaria protection.
Before evaluating RTS,S/AS01 in large-scale trials we assessed whether
the vaccine, administered with and without antimalarial drugs, is safe and
immunogenic. The study recruited 193 healthy adult volunteers randomized
to 7 arms of variable regimens of RTS,S with and without the MDA regimen
(dihydroartemisinin-piperaquine) plus primaquine. One month after the last
vaccination all study participants had seroconversion of the PfCSP (NANP)6 and
the PfCSP Full Length (N + C-Terminal). More than 90% of vaccine recipients had
seroconverted to the Pfanti-C-Term CSP. All studied subjects were followed for
6 months. The results showed no drug-vaccine interactions. Neither was there a
relevant change of the drug concentrations nor the antibody levels when vaccine
and antimalarial drugs were combined. This study found that RTS,S/AS01 with
and without dihydroartemisinin-piperaquine plus single low dose primaquine is
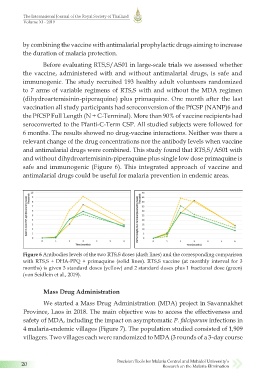
safe and immunogenic (Figure 6). This integrated approach of vaccine and
antimalarial drugs could be useful for malaria prevention in endemic areas.
Figure 6 Antibodies levels of the two RTS,S doses (dash lines) and the corresponding comparison
with RTS,S + DHA-PPQ + primaquine (solid lines). RTS,S vaccine (at monthly interval for 3
months) is given 3 standard doses (yellow) and 2 standard doses plus 1 fractional dose (green)
(von Seidlein et al., 2019).
Mass Drug Administration
We started a Mass Drug Administration (MDA) project in Savannakhet
Province, Laos in 2018. The main objective was to access the effectiveness and
safety of MDA, including the impact on asymptomatic P. falciparum infections in
4 malaria-endemic villages (Figure 7). The population studied consisted of 1,909
villagers. Two villages each were randomized to MDA (3 rounds of a 3-day course
20 Precision Tools for Malaria Control and Mahidol University’s
Research on the Malaria Elimination
11/7/2565 BE 13:27
_22-0424(011-026)3.indd 20
_22-0424(011-026)3.indd 20 11/7/2565 BE 13:27