Page 23 - 22-0424
P. 23
The International Journal of the Royal Society of Thailand
Volume XI - 2019
(TACTs) in which a third already licensed antimalarial drug is added to the existing
co-formulated ACT. Each study recruited over a thousand patients and the total
patients of both studies were 2,341 adults and children with uncomplicated
falciparum malaria. The results of these 2 studies revealed that artemisinin-resistant
P. falciparum has not been contained and is prevalent across mainland Southeast
Asia (Ashley et al, 2014). Artemisinin resistance is associated with mutations in
kelch 13 and an increased transmission potential. The triple drugs now called TACTs
(dihydroartemisinin–piperaquine plus mefloquine or artemether–lumefantrine
plus amodiaquine) are efficacious, well-tolerated, and safe for uncomplicated
P. falciparum malaria, including in areas with resistance to artemisinin and ACT
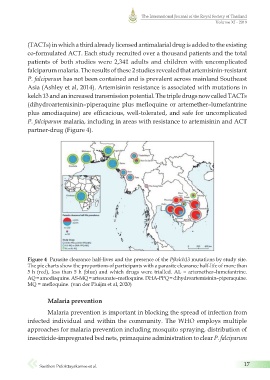
partner-drug (Figure 4).
Figure 4 Parasite clearance half-lives and the presence of the Pfkelch13 mutations by study site.
The pie charts show the proportions of participants with a parasite clearance half-life of more than
5 h (red), less than 5 h (blue) and which drugs were trialled. AL = artemether–lumefantrine.
AQ = amodiaquine. AS-MQ = artesunate–mefloquine. DHA-PPQ = dihydroartemisinin–piperaquine.
MQ = mefloquine. (van der Pluijm et al, 2020)
Malaria prevention
Malaria prevention is important in blocking the spread of infection from
infected individual and within the community. The WHO employs multiple
approaches for malaria prevention including mosquito spraying, distribution of
insecticide-impregnated bed nets, primaquine administration to clear P. falciparum
17
Sasithon Pukrittayakamee et al.
11/7/2565 BE 13:27
_22-0424(011-026)3.indd 17
_22-0424(011-026)3.indd 17 11/7/2565 BE 13:27