Page 105 - 22-0424
P. 105
The International Journal of the Royal Society of Thailand
Volume XI - 2019
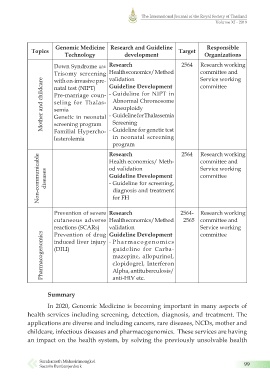
Genomic Medicine Research and Guideline Responsible
Topics Target
Technology development Organizations
Down Syndrome และ Research 2564 Research working
Trisomy screening Health economics/ Method committee and
validation
Service working
with on-invasive pre-
Mother and childcare Pre-marriage coun- - Guideline for NIPT in
Guideline Development
committee
natal test (NIPT)
Abnormal Chromosome
seling for Thalas-
Aneuploidy
semia
- Guideline for Thalassemia
Genetic in neonatal
Screening
screening program
Familial Hypercho-
in neonatal screening
lesterolemia - Guideline for genetic test
program 2564 Research working
Research
Non-communicable diseases od validation Service working
Health economics/ Meth-
committee and
committee
Guideline Development
- Guideline for screening,
diagnosis and treatment
for FH
Prevention of severe Research 2564- Research working
cutaneous adverse Health economics/ Method 2565 committee and
reactions (SCARs) validation Service working
committee
Pharmacogenomics induced liver injury - Pharmacogenomics
Prevention of drug Guideline Development
guideline for Carba-
(DILI)
mazepine, allopurinol,
clopidogrel, Interferon
Alpha, antituberculosis/
Summary anti-HIV etc.
In 2020, Genomic Medicine is becoming important in many aspects of
health services including screening, detection, diagnosis, and treatment. The
applications are diverse and including cancers, rare diseases, NCDs, mother and
childcare, infectious diseases and pharmacogenomics. These services are having
an impact on the health system, by solving the previously unsolvable health
Surakameth Mahasirimongkol
Sacarin Bunbanjerdsuk 99
11/7/2565 BE 13:30
_22-0424(089-102)9.indd 99 11/7/2565 BE 13:30
_22-0424(089-102)9.indd 99